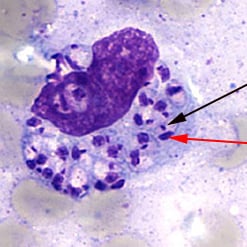
1) Pentavalent Antimonials – Serious cardiotoxicity is seen in 9-10% of patients. Other side effects are reversible renal insufficiency, pancreatitis, anemia, leukopenia, rash, headache, abdominal pain, nausea, vomiting, arthralgia, myalgia, thrombocytopenia, and transaminase elevation. Effectiveness of treatment increases with longer courses of treatment, however incidence of side effects does as well.
2) Amphotericin B – Infusion-related adverse events can occur with amphotericin B deoxycholate. Toxicities which can be seen are hypokalemia, nephrotoxicity and myocarditis. Liposomal amphotericin B is much safer, with fewer side effects.
3) Miltefosine – Gastrointestinal side effects are the most common and include vomiting. Transient elevation of hepatic enzymes has been observed. The drug is highly teratogenic and as such is contraindicated in pregnant patients and mandatory contraception is recommended for women of child-bearing age through treatment and 2-3 months after.
4) Paromycin – Injection-site pain was the most frequently reported side effect in one study done in India. Ototoxicity and nephrotoxicity are side effects that are common in aminoglycoside antibiotics, however frequency at therapeutic doses is low. Monitoring of hepatic enzymes has been recommended.
5) Pentamidine – The major side effect of pentamidine use is induction of insulin-dependent diabetes mellitus. It a major concern and one of the reasons it is a second line treatment. Other side effects include nausea, vomiting, abdominal pain, and headache.
For more information:
www.rxlist.com
Seifert, K., (2011). Structures, targets and recent approaches in anti-leishmanial drug discovery and development. The Open Medical Chemistry Journal, (5), 31-39.